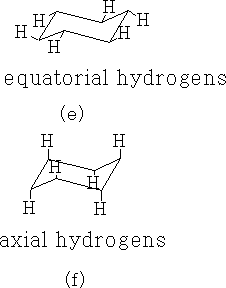
There are different types of isomerism
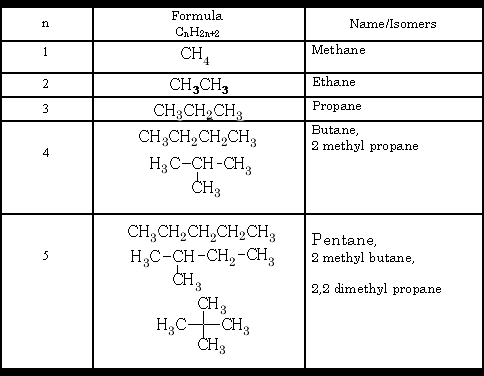
a) Chain – carbon skeletons
b) Position or geometrical – chemically similar, same functional group
c) Functional group – functional group is different for each compound
d) Optical – ability to rotate polarised light.
Chain isomerism is seen in the alkenes:

The carbon chain can be arranged in different ways, each one is different in its physical properties.
Position isomerism is seen in the alkenes:
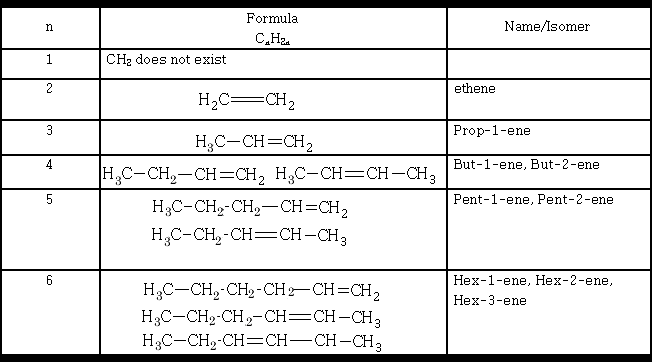
The isomers mentioned in the table above by no means represent the total number of isomers that exist for the alkenes shown, can you write the others?
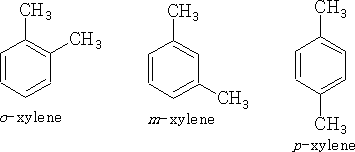
Position isomerism can also be seen in Benzene compounds:
Functional group isomerism comes about because a formula which is written can be ambiguous e.g.:
![]() can be either
can be either ![]() or
or ![]() the first is ethanol the second dimethyl ether.
the first is ethanol the second dimethyl ether.
Optical Isomerism
If we pass a beam of light through a polariser what we get is a beam which vibrates in a single plane rather than in all directions in a normal beam. This beam is said to be plane-polarised. Certain substances have the ability to rotate the beam of plane-polarised light. Compounds that do this are said to be optically active. If the beam of plain polarised light is rotated in a clockwise direction as viewed from the direction of the emergent beam, the rotation is given the designation (+); an anticlockwise direction is designated (-).
What type of molecule will rotate the plane of polarisation? The answer is an asymmetric molecule i.e. one that has 4 different groups attached to a central carbon atom, consider: a) and b) below:
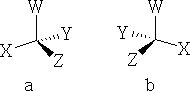
The two molecules are indeed asymmetric and they have something else in common! They are mirror images of one another. Compounds like this are said to be chiral . The thing that makes them different compounds is that the mirror images are non-superimposable. These arrangements are called enantiomers or stereoisomers . e.g.
Ernest L. Eliel and Samuel H. Wilen (1994). The Sterochemistry of Organic Compounds . Wiley-Interscience.
http://www.daviddarling.info/encyclopedia/E/enant.html
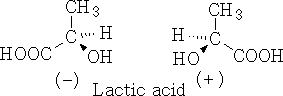
(-) Laevo-rotatory and (+) dextro-rotatory
Therefore the molecules (-) Lactic acid rotate the light anticlockwise and (+) Lactic acid rotates the light clockwise.
Systems with more than one asymmetric centre e.g. tartaric acid will give a combination of enantiomers.
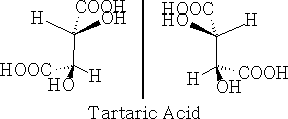
a) non-superimposable Mirror images
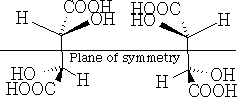
b) superimposable images with plane of symmetry
Firstly, in a) above, they are non-superimposable mirror images and hence are optically active, and in b) the forms are superimposable mirror images and have a plane of symmetry so are the same compound and are not optically active. This version is called meso-tartaric acid. The reason that this pair of compounds are not optically active is that they have this plane of symmetry. Build these molecules and convince yourself that they truly are superimposable.
In general, n asymmetric centres give 2n -1 pairs of enantiomers.
There are different ways in which the molecules can be represented: e.g.
Consider meso-tartaric acid
We can have:
1 Flying wedge projection as seen above:
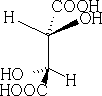
2 Saw-horse projection:
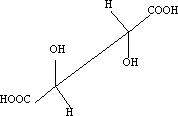
3 Newman projection:
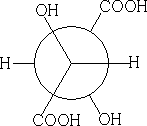
4 Fischer Projection:
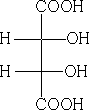
In the Fischer convention the horizontal bonds are coming forward out of the paper, the vertical ones going back behind.
Isomerism associated with cyclic compounds
1 Cyclopropanes:
The cyclopropanes lie in one plane and can be substituted as follows:
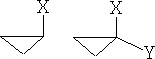
a)
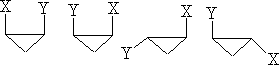
These compounds can be mono or di-substituted; the forms shown in a) can only exist in one form. However, figure b) shows di-substituted compounds which can exist in a number of forms, in fact, four,
Absolute Configuration
How can we denote the exact arrangement of groups around a stereocentre? This exact arrangement is called the absolute configuration, we can use a system that was developed by Cahn-Ingold-Prelog and is called the CIP notation, and it provides an unambiguous description of a stereoisomer of a compound with stereocentres.
There are two possible absolute configurations around any single stereocentres and the configurations will be given the notation R and S under the CIP notation.
The CIP notation has a set of rules and it will require that you have also a good sense of spatial awareness .
Below are some group priorities that will be useful when making decisions about where groups come in the hierarchy.
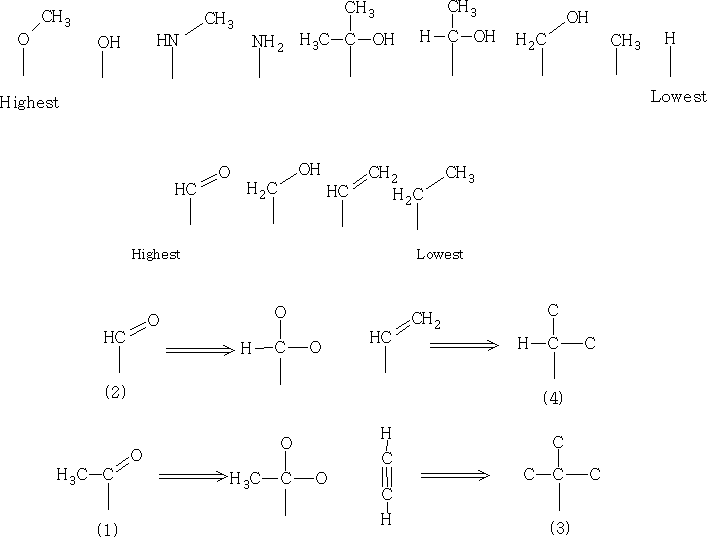
To find the absolute configuration we will need to look at the tetrahedral stereocentre and examine the exact orientation of the groups relative to each other.
The thing to look at is the AMU of the groups attached. Two identical groups have to be looked at carefully and to decide on the rank of the highest priority substituent on each. The list above shows how the priority is set out and shows also the priority of multiple bond substituents. In multiple bond substituent's we count each bond as a separate connection. So ethene is higher priority than an alkane.
Consider:

We can see the configuration S and R have been designated. How is this done?
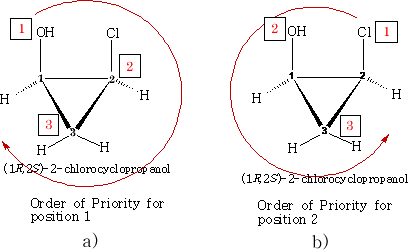
If we consider a) the position 1 has an oxygen atom attached, two carbons and one hydrogen. In terms of priority the oxygen must be number 1; as there are two carbon atoms we need to look at what is on each carbon to determine which comes next in the order of priority. Carbon 2 has a chlorine atom attached and carbon 3 has only two hydrogens attached. Carbon 2 must be the next priority. Carbon 3 is therefore third in the order because hydrogen has to be last. The priority is then clockwise which makes it 1R .
If we now consider b) we need to look at the priorities with respect to carbon 2. Using the same logic the priorities come out anticlockwise hence position 2 is 2S .
Exactly the same can be done for c) and d) below, work it out for yourself' confirm the configurations.

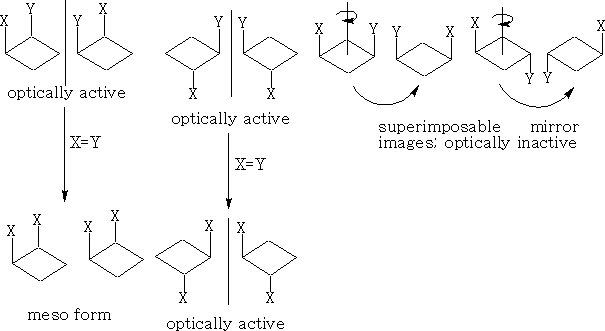
If x=y then the two cis enantiomers contain a plane of symmetry and become meso types.
2 Cyclobutanes; There is a similarity between these and the cyclopropanes, except the ring is slightly more strained and tends to be a little buckled. Also, because of the increased number of carbon atoms we now have the possibility of 1, 3 di-substituted compounds.
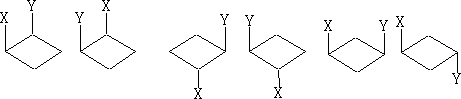
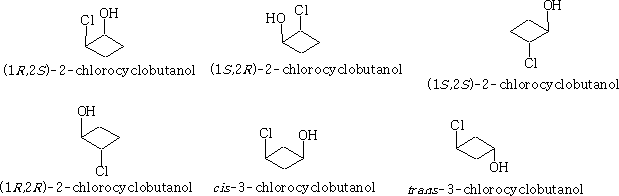
With these 1, 2 di-substituted compounds they exist as diastereoisomers or a pair of enantiomers and a meso form if x=y. The 1, 3 compounds both contain a plane of symmetry and are not optically active.
3 Cyclopentanes
The cyclopentane adopts the ‘envelope' structure because of the opposing strain of the angular component and the eclipsing factor. The 1, 2 and 1, 3 compounds have four forms, two diastereoisomers for each cis and trans variety.
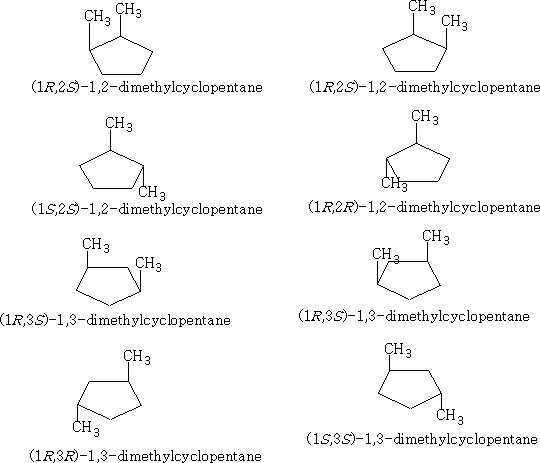
4 Cyclohexanes:
The cyclohexanes have the least strain of all the cyclo compounds; they can adopt a structure that is free from angular and conformational strain.
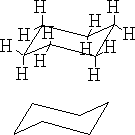
a)
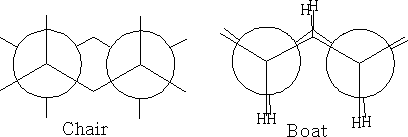
The structure (a) shows this conformation, it is known as the chair structure. It can flip into its mirror image (b).
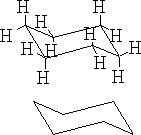
b)
This flipping occurs rapidly at room temperature as the energy barrier between forms is 42kJ mole-1 .
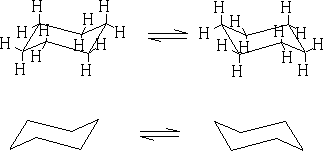
In between these two structures there are two other forms which the structure goes through: the twist boat (c) and the boat conformation (d). The twist boat is 23kJ mole-1 less stable than the chair and the boat conformation which is 30kJ moles-1 less stable.
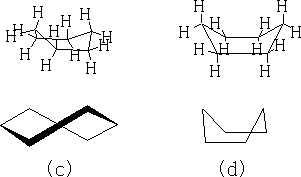
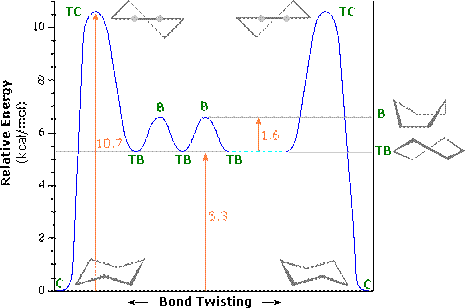
Table 1
Table 1 shows the energy profile of the conformation change for cyclohexane. We can see that the twist boat is more stable than the boat form; this is because there are some eclipsing interactions with the boat form.
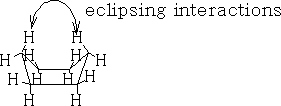
The interactions can be better seen if we look at the Newman projections of cyclohexane:
The C-H bonds in cyclohexane are of two types, axial (f) and equatorial (e).