Organometallic compounds are as they sound a carbon containing molecule attached to a metal. The commonest metals are Mg, Li, Na, Hg, Cd and Zn.
Polarisation is apparent in the molecule:
R2Cδ- Mδ+, it varies from a not very polar covalent system to almost fully ionised species.
R2C - is called a carbanion. By definition, every carbanion possesses an unshared pair of electrons and is therefore a base. When it accepts a proton, it is converted into its conjugate acid.
The stability of the carbanion is related to the strength of the conjugate acid, the weaker the acid, the stronger the base and lower the stability of the carbanion. By stability we mean stability toward a proton donor: the lower the stability the greater likelihood the carbanion will pick up a proton from an available source.
Nomenclature:
Alkyl metal, e.g. tetraethyl lead; Alkyl metal salt e.g. ethyl magnesium bromide
Chemical properties
Characteristically, the reactions are of nucleophilic carbon regardless of whether the organometallic is ionised or not.
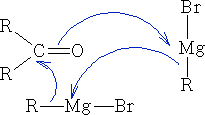
Most typically:
![]()
![]()
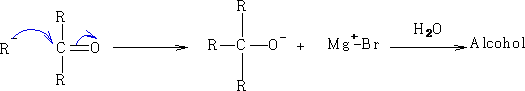
Reactions of Grignard reagents; RMgHal e.g. EtMgBr (ethyl magnesium bromide)
1 Et- as a strong base, reacting with proton acids:
![]()
![]()
![]()
![]()
2 Reactions with electrophilic carbon:
a)
![]()
b)
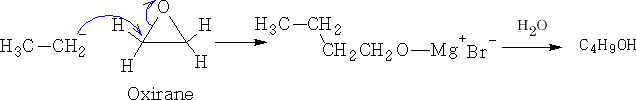
c) Aldehydes and ketones.
Et- with CH2O ![]() 1o alcohols CH3CH2OH
1o alcohols CH3CH2OH
Et- with RCHO![]() 2o alcohols CH3CH2(R)CHOH
2o alcohols CH3CH2(R)CHOH
Et- with R2CO![]() 3o alcohols CH3CH2(R)2COH
3o alcohols CH3CH2(R)2COH
d) Esters and acid chlorides (X=Chloride)
![]()
![]()
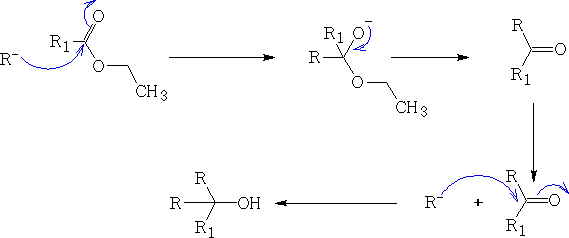
As you can see from the above examples once the Grignard reagent has been let lose it will continue to react to form the tertiary alcohol.
e) With carbon dioxide
![]()
Try to work out the mechanism for this reaction .
f) With Nitriles
In this reaction the ketone is produced because generally the Grignard reagent is destroyed before further reduction can occur.
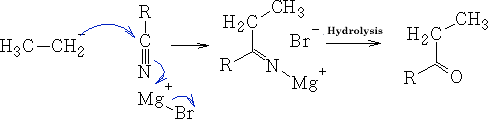
The reaction goes via the imine R2C=NH.
3 Reactions with other electrophiles.
a) With oxygen
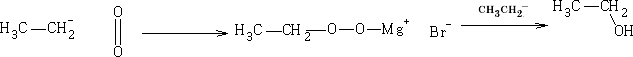
b) With bromine
![]()
All acids have a conjugate base, and all bases have a conjugate acid.