Nomenclature of the Halogenalkanes: CnH2n+1X
These are compounds that contain halogen atom in place of one of the hydrogen's in an alkane.
Halogenalkanes are:
Primary RCH2 -X 1o
Secondary R2CH-X 2o
Tertiary R3C-X 3o
Halogens are electronegative elements, and the molecules have dipoles. When attached to an alkane a dipole will be set up with the less electronegative carbon.
The small positive charge on the carbon atom makes it susceptible to attack by a group with a lone pair of electrons.
These groups are called nucleophiles, i.e.
![]()
A : is the nucleophile and B will in this case be an electrophile . Consider the following; say whether they are electrophile or nucleophile.
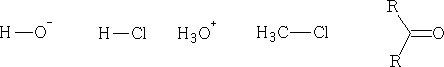
Some electrophiles are positively charged cations abbreviated E+, and Nucleophiles are negatively charged anions usually abbreviated Nu- (not always true).
The bpt's are often more or less close to that of the hydrocarbon of the same molecular weight.
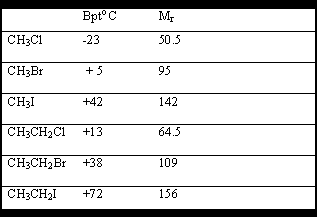
Halogenalkanes undergo various types of reactions a) nucleophilic substitution Sn1&Sn2 and b) elimination E1 & E2
Both types of reactions will be covered later as a separate item.
The size of the δ+ on the carbon atom adjacent to a halogen group can have an effect on the reactivity of the compound towards nucleophiles.
• if the halogen is more electronegative, the δ+ will be larger.
• if there are more halogens on the C atom the δ+ will be larger.
• if there are more alkyl groups (CH3 ,C2H5 etc) the δ+ will be smaller, because alkyl groups are weakly electron releasing.
Note also that the strength of the bond being broken in the reaction is important in determining how quickly reactions happen.
Strengths of bonds are compared using bond energies:
With aqueous OH-
![]()
The alcohol is formed.
With ammonia (dissolved in alcohol) an amine is formed.
![]()
With cyanide (NaCN in alcohol) it forms a nitrile.
![]()
This reaction is important as it is a method used to increase the carbon chain.
With alcoholic KOH an alkene is formed.
![]()
Preparation of halogenalkanes
1. Addition of HX or X2 to an alkene
![]()
*see Markownikovs rule in the addition of an acid to the carbon-carbon double bond of an alkene, the hydrogen of the acid attaches itself to the carbon that already holds the greater number of hydrogen's.
![]()