The Diels-Alder reaction was first discovered by Teacher and pupil Otto Diels and Kurt Alder. They won a Nobel Prize for chemistry in 1950 for their work. The reaction is a 4π + 2π electron cycloaddition reaction. What is formed is a cyclohexene compound. e.g.

The reaction shown is not a very favourable reaction as the activation energy for the reaction is very high, however, the reaction can be made more favourable by adding substituents to both the diene and the alkene.
If we consider the Molecular orbitals we can see how the reaction can work.
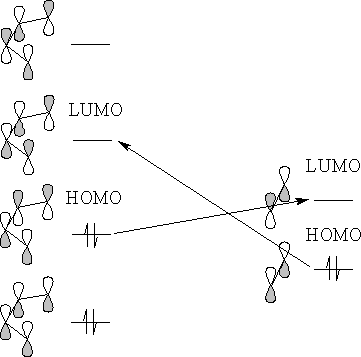
There are two possible interactions, the HOMO of the diene with the LUMO of the alkene or the HOMO of the alkene with the LUMO of the diene. Since the HOMO of the diene has the highest electron energy the reaction will be the HOMO of the diene with the LUMO of the alkene.
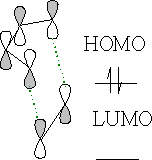
The orbitals line up perfectly with the correct configuration.
If we add one or more Electron Donating Groups (EDG) to the diene we can make it more electron rich, e.g.
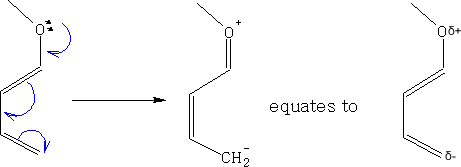
The alkene is refered to as a dienophile and this can be enhanced by adding one or more Electron Withdrawing Groups (EWG) to make it electron poor. e.g.
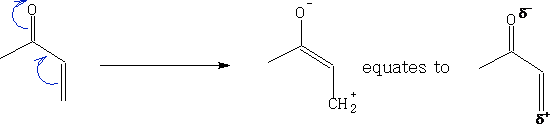
These forms will react more easily, putting these together we get:
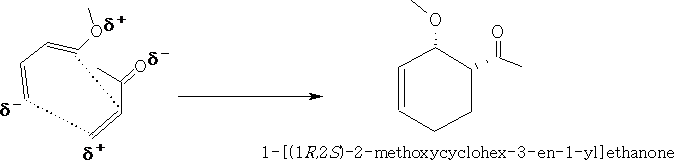
Looking at the molecular orbitals we can see how the molecules interact.
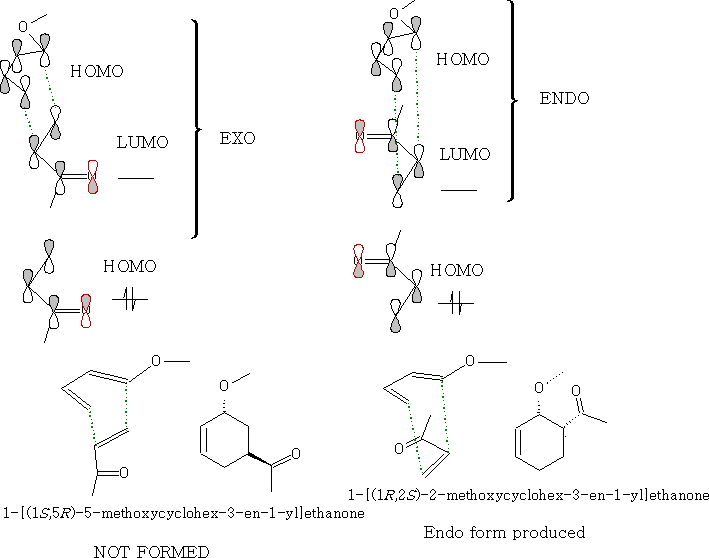
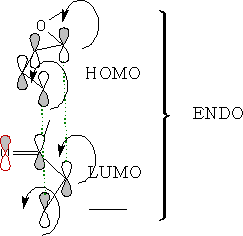
As the molecules approach each other they rotate to form the ENDO form:
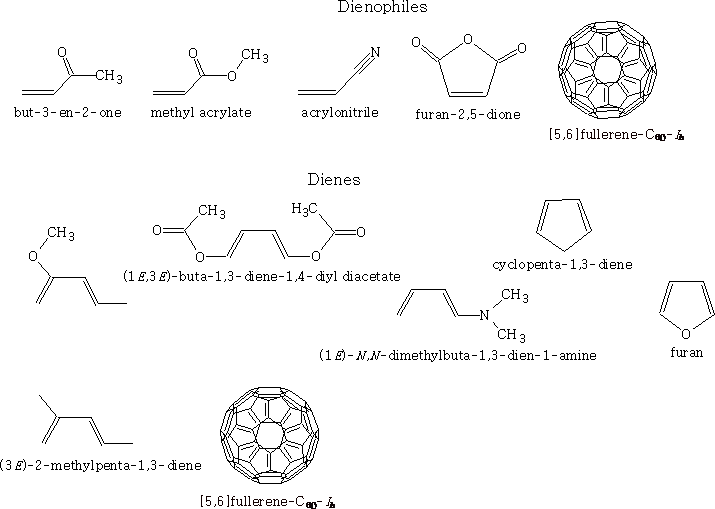
Here are some examples of dienes and alkenes with groups attached: