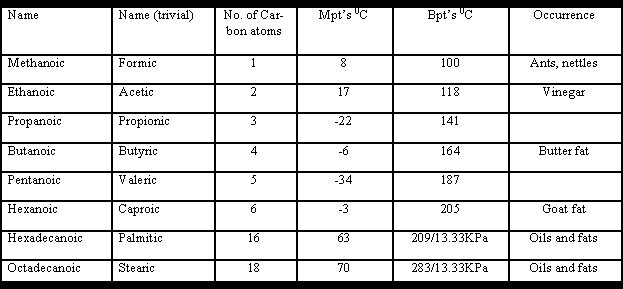
Nomenclature:
The names end in –oic although many trivial names are still used. In this text I will stay with the systematic names with maybe the occasional reference to the trivial name in brackets.

Numbering system:
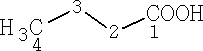
Butanoic acid
The carbons are numbered from the -COOH group, with that taking position 1.
Physical Properties
The aliphatic acids have high mpt's and bpt's due mainly to the strong hydrogen bonding:
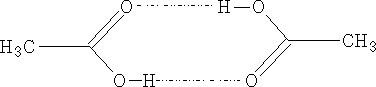
The acids to a certain extent exist as dimmers even in the vapour state.
They have a pungent odour, falling off as the series ascends up to C4, they are miscible with water.
The aromatic acids are almost always solids, with little or no smell; they are only slightly soluble in water.
Preparation of Carboxylic acids:
1. By oxidation:

In the laboratory this reaction is usually done using K2SO4 /H2SO4 or KMnO4 / OH-. Industrially, oxidations are carried out at high temperature and pressure, using various metals as catalyst, or even oxygen.
2. Aromatic side-chain
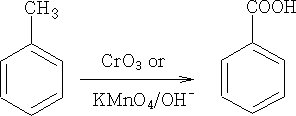
3. By hydrolysis of nitriles:
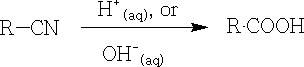
The use of acid or alkali is both valid, but the former is preferred. This is an important procedure, since nitriles are easily prepared.
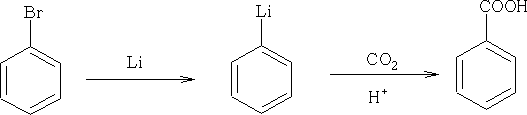
4. From Organometallics:
The use of Grignard reagents is an important method. The general reaction:
![]()
This reaction uses carbon dioxide with hydrolysis with acid to form the
–COOH group.
Lithium compounds are also used widely:
5. From malonic esters:
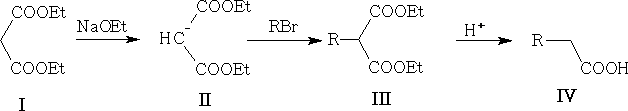
This approach can be used to prepare disubstituted acids, e.g.
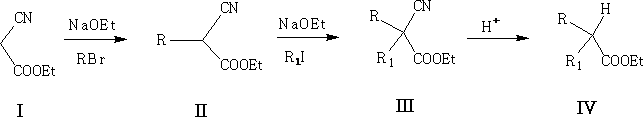
This reaction is the same as the previous; here the CN group is added and removed at the end by hydrolysis. Try to work out the reaction mechanism for III to IV.
Derivatives of Carboxylic acids
a. Salts: High mpt's. Ionised; water soluble. RCOOM
b. Acid Halides: Only the chlorides are important. These are made from the acids and PCl3 , PCl5 , SOCl2 , or COCl2 . The halides are easily hydrolysed and will fume in damp air. RCOX
c. Anhydrides: Made from the salt and the acid chloride. These are less reactive than the acid halides. (RCO)2O
d. Esters: Many of the esters have characteristic sweet smells; they are usually liquids: General methods of preparation include: RCOOR'
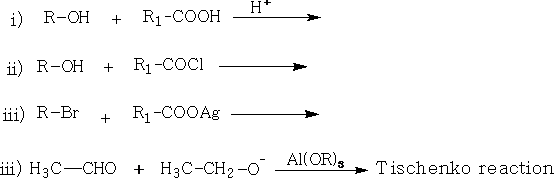
Tetrahedron Letters 2003, Vol 44, # 15 p. 3191 - 3194
5. Amides: These are usually high mpt solids. General methods of preparation include:
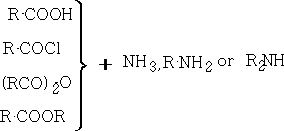
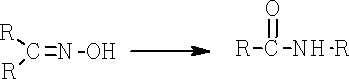
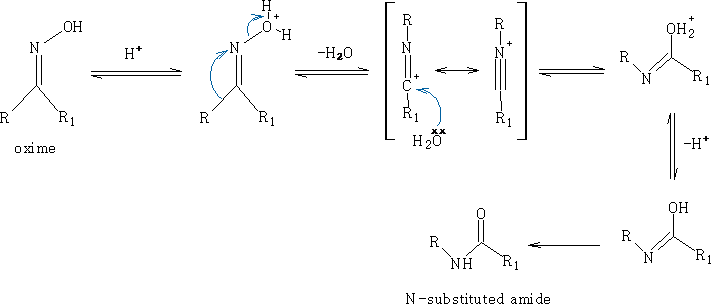
ii) Beckmann rearrangement:
R. K. Hill, O. T. Chortyk, J. Am. Chem. Soc. 84, 1064 (1962)
Mechanism
iii) Partial hydrolysis of nitriles:
![]()
6. Hydrazides: Usually prepared by reacting an ester with the appropriate hydrazine: RCONHNH2 , RCONHNHR1, RCONHNR1R2 e.g.
![]()
7. Hydroxamic acid. Prepared by the reaction of an ester with hydroxylamine: RCONHOH
![]()
8. Imides: This is the nitrogen analogue of the anhydride; (RCO)2NH generally prepared by heating a compound containing both an acid and an amide. e.g.
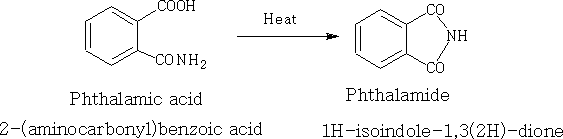
9. Peracids: Normally prepared by oxidation of the carboxylic acid with hydrogen peroxide. RCOOOH is used in the production of:
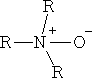
i) Amine oxides
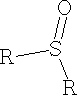
ii) Sulphoxides e.g. DMSO Dimethyl sulphoxide
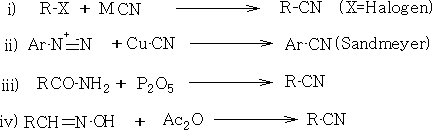
10. Cyanides: There are many methods for preparation.
Reactions of Acids and their Derivatives
i) Electrophilic attack on oxygen; as with aldehydes and ketones the most important reaction is protonation:
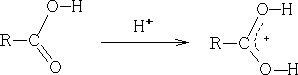
With derivatives there are two possible sites for protonation, e.g.