Carbohydrates have the general formula of Cx(H2O)y and are called hydrates of water because they contain hydrogen and oxygen in the same proportions as that of water. Obviously not all substances with the general formula Cx(H2O)y are necessarily carbohydrates, e.g. formaldehyde CH2O; ethanoic acid CH3COOH; carbohydrates are polyhydroxy aldehydes or ketones or will give these on hydrolysis.
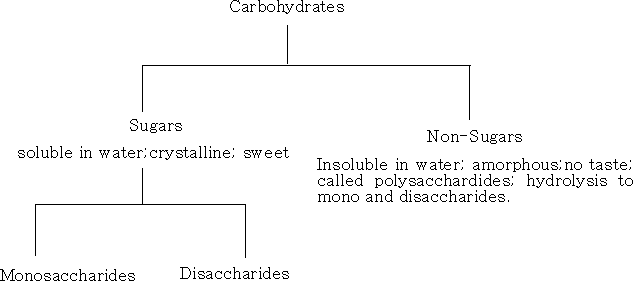
Carbohydrates usually have the - ose ending; aldehydic structures are aldoses, and ketonic structures are ketoses. There are two main classes of carbohydrate, sugars and polysaccharides.
Sugars are sub-divided into:

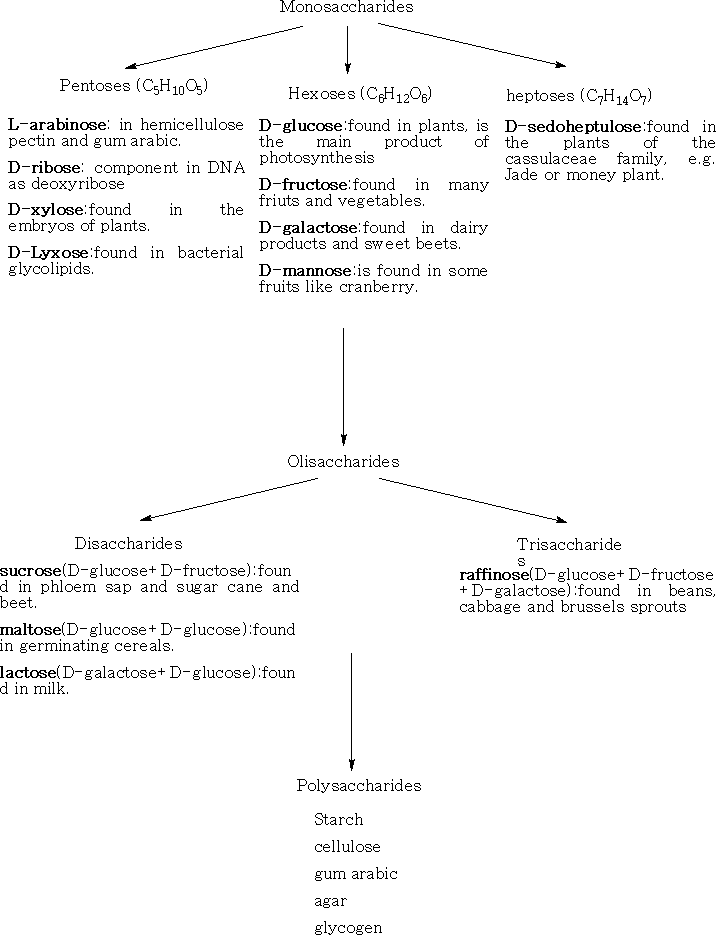
1 Monosaccharide's; these cannot be hydrolysed into smaller molecules. They have the formula CnH2nOn , where n= 2 - 10. The most important are the pentoses and the hexoses which occur naturally in plants.
2 Oligosaccharide's; these yield 2 – 9 monosaccharide's on hydrolysis.
‘Sugars are optically active polyhydroxy-aldehydes and ketones' my consideration at this time will be with the pentoses and the hexoses.

also;
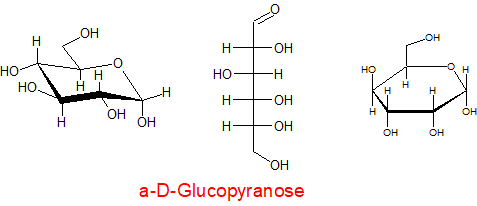
The carbohydrates can be drawn in many ways, e.g. Fisher projections
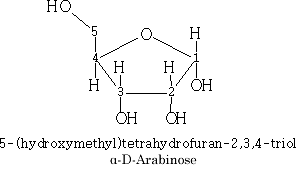
Pentoses: C5H10O5
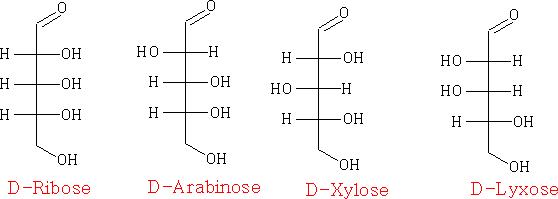
in the Haworth projection:
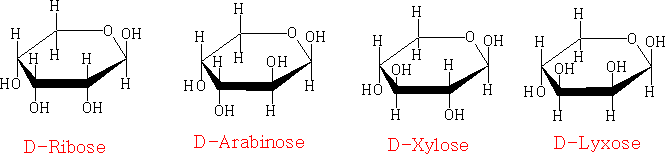
the chair projection:
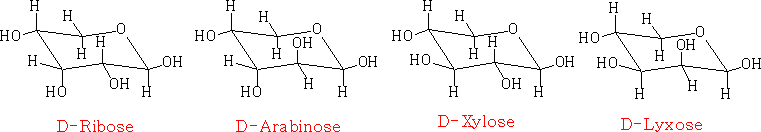
the Fischer projection joined as a ring:
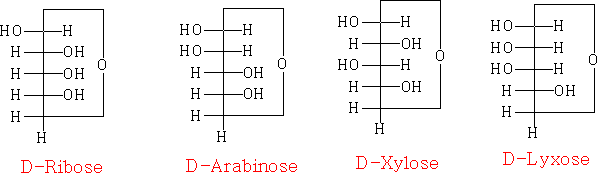
In each of the above structures the carbon which is designated as position 1 can have two conformations α or β. The α position is where the OH group is down with respect to the ring e.g.
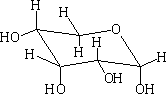
α-D-ribopyranose
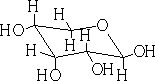
β-D-ribopyranose
In solution these forms will mutarotate from the α form to the ß form and visa versa.
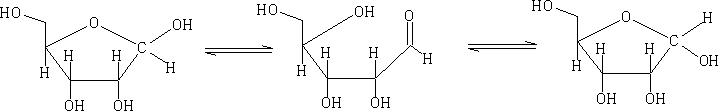
The pyranose structures are more stable then the furanose form for the free sugar. The ribose structure is shown as the furanose form because it is this form that is found in nature.
The common six-carbon sugars (Hexoses) are D-Glucose, D-Fructose, D-Galactose, and D-Mannose