Aromatic Amines
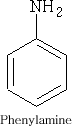
The simplest aromatic amine is aniline; Phenylamine
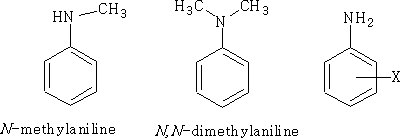
Nomenclature:
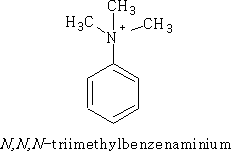
As well as the above examples there are also the quaternary ammonium salts of the form:
Preparation of Phenylamine:
This is prepared by the catalytic hydrogenation with hydrogen of nitrobenzene:
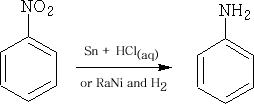
Chemical properties
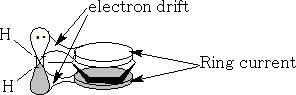
The amine in the aromatic system is less basic than in the aliphatic amines. This is because the ring current tends to pull the electrons away from the nitrogen into the ring and makes them less available for protonation.
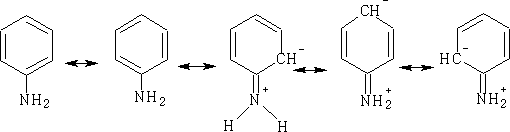
Can write these forms as:
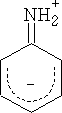
The basicity of the aromatic Phenylamine is much smaller:
Phenylamine pKBH+ = +4.6 compare cyclohexylamine (C6H11NH2) pKBH+ = +10.6
The basicity of the aromatic amines is dependant on the substituent group. e.g. 4-nitro phenylamine
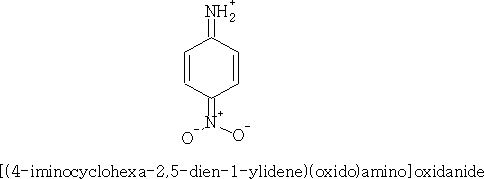
We can see that this is an even weaker base; this is because the NO2 group pulls more electrons away from the NH2 group making protonation even harder.
Consider; 4-methyl phenylamine
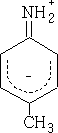 What will be the effect of the methyl group?
What will be the effect of the methyl group?
pKBH+=+5.08
Basicity has been enhanced; the methyl group pushes electrons into the ring.
Effect of Nitrogen on the SEAr
We have seen already that the Wheland intermediate is stabilised by the amine group, and that it will direct reactions toward the 1,4 positions on the ring.
1 Halogenation: Cl2 and Br2 will react fast to give:
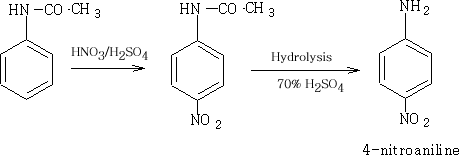
To obtain a mono-substituted product the NH2 group must be deactivated. This is done by adding ethanoyl chloride to the phenylamine.
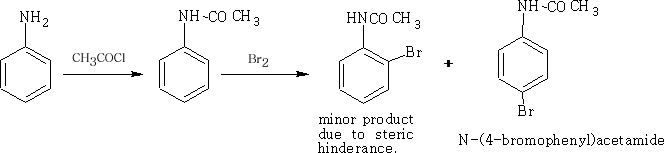
The –COCH3 group has the effect of slowing the donation of the lone pair of electrons on the N atom by the following reaction:
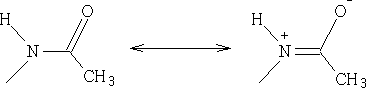
2 Nitration:
If we use phenylamine it will be oxidised quite readily, however, the acetylated amine will give the product we require.
Nitration of the benzenaminium ion slowly gives the 3-substituted product:
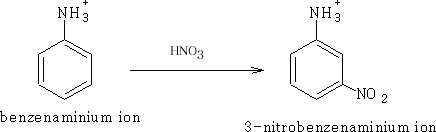
The –NH3+ has a tendency to deactivate the ring and so allow the -NO2 group to add in the 3-position.
3 Sulphonation:
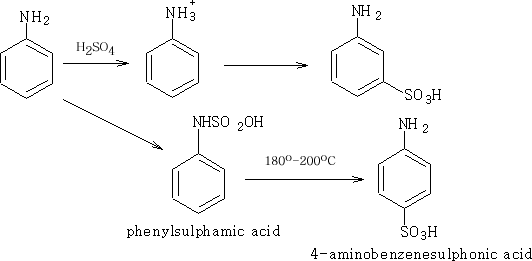
This reaction produces two different products, the 3-aminobenzenesulphonic acid when the reaction is performed on the benzenaminium ion and the other when the phenylsulphamic acid is heated at 180o-200oC. The products are high melting point solids, and insoluble in water. This is unexpected since benzenesulphonic acid has a melting point of 66oC and is very soluble in water.
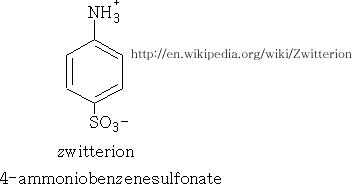
The 4-aminobenzenesulphonic acid is a salt known as a dipolar ion or a zwitterion. It is a reaction of the acidic part of a compound reacting with the basic part of the same compound; it occurs more commonly in amino acids.