
Chemistry is all about getting lucky
- Robert Curl Nobel Prize for Chemistry 1996
Benzene is the first of the ‘aromatics'. Formula C6H6
It is a planar, cyclic molecule with all C-C bonds equal, at 140 pm:
Compare C – C in (sp3 – sp3 hybridised) alkanes of 154 pm and 134 pm in C=C (sp2 – sp2 hybridised) alkenes. The bond is longer than C=C but shorter than C –C indicating a different structure.
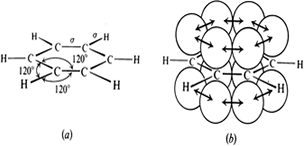
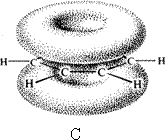
Benzene rings a) with s bonds b) p orbital overlap c) p clouds above and below ring
Here we see the delocalisation of the electrons in a ring from the overlap of the sp2 hybridised orbitals, a consequence of having alternate double and single bonds (conjugation). Conjugated bonds have different properties from the isolated double bonds. In terpenes you will meet ß-carotene:
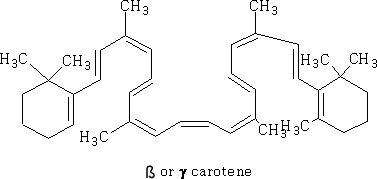
This has a large number of double and single bonds, all of the sp2 orbitals can overlap to form a conjugated system. One of the consequences of this conjugation is that this molecule has colour.
In the conjugated ring that is benzene a ring current is set up as the electrons are able to move through the structure. X-ray and electron diffraction show benzene to be a completely flat molecule with all C – C bonds being equal and all angles to be 120o.
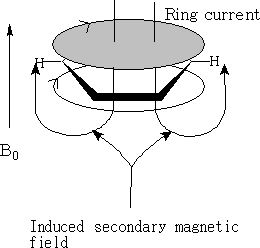
With NMR on applying a magnetic field the ring current induces a secondary magnetic field. The magnetic field felt by the –H on the ring is much greater. The proton is pushed to low field. NMR for the aromatic proton is δ 7.3.
The structure of benzene can be represented as:
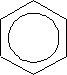
This shows the ring current covering the 6 carbon atoms or actually means the hybrid of the Kekule structure:
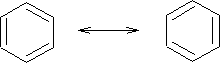
This effect is called mesomerism .
Mesomerism. The condition exhibited by a molecule when the actual arrangement of its valence electrons is intermediate between two or more arrangements having nearly the same energy, and the positions of the atomic nuclei are identical.
Consider cis 2-butene; Hydrogenation gives:
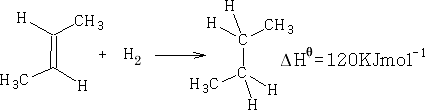
If Benzene were just 3 C=C double bonds, then;
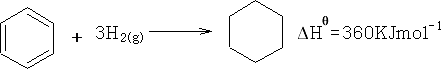
But with actual benzene:
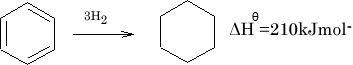
The difference is called the Resonance energy of benzene. The stability of benzene is greater by 150KJ mol-1 .
It is not my intention to go into the reasons behind the aromaticity but if you wish to investigate further then look at Huckle's Rules for aromaticity.
Nomenclature : Arenes
C6H6 Benzene
C6H5CH3 Toluene (methyl benzene)
C6H4(CH3)2 Xylenes (1,2 – 1,3 – 1,4 dimethyl benzene)
The groups become:
C6H5 - Phenyl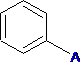
C6H5CH2 - Benzyl 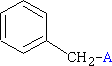
CH3C6H4 - Tolyl 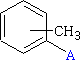
In benzene the numbering is as follows, the main functional group taking position 1.
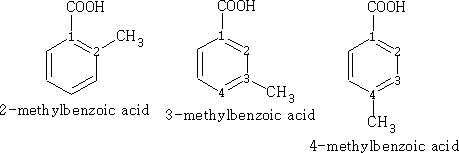
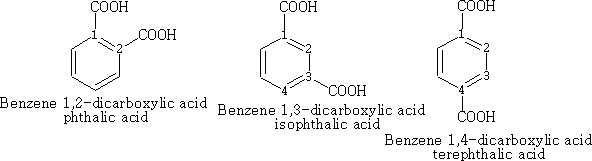
Chemical properties
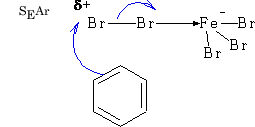
The electrophile needs to be activated because attack involves the disruption of the benzene ring.
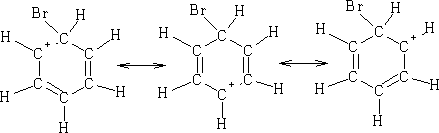
This is the Wheland Intermediates1and can be written:
1. A Quantum Mechanical Investigation of the Orientation of Substituents in Aromatic Molecules G. W. Wheland J. Am. Chem. Soc. ; 1942 ; 64(4) pp 900 - 908;
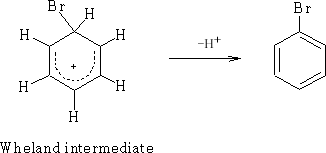
In general E+ will add via the Wheland intermediate and is called aromatic electrophilic substitution (SEAr).
Some electrophiles:
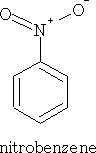
1 Nitric acid: Dioxoammonium ion
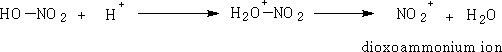
This reaction is facilitated by mixing the nitric acid with conc. H2SO4.
![]()
The reaction proceeds as for Br2 adding to the benzene ring to give:
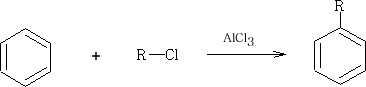
2 With RCl with a catalyst of AlCl3
 .
.
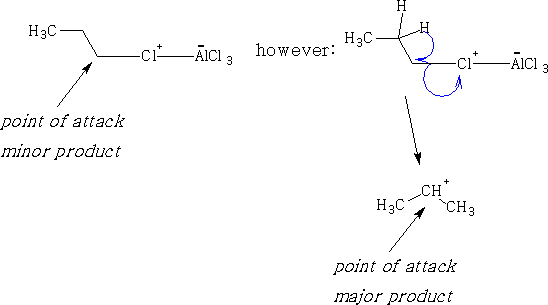
This reaction cannot be used with primary alkyl halides larger than ethyl chloride, as we obtain a number of by-products. Primary cations are not stable in solution and a rearrangement occurs in the primary alkyl halide.
e.g. CH3CH2Cl gives;
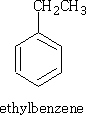
This reaction is called the Friedel-Crafts2 alkylation. However, propyl chloride gives:
2 G. A. Olah, Friedel-Crafts and Related Reactions, 3 volumes (New York, 1963-1965).
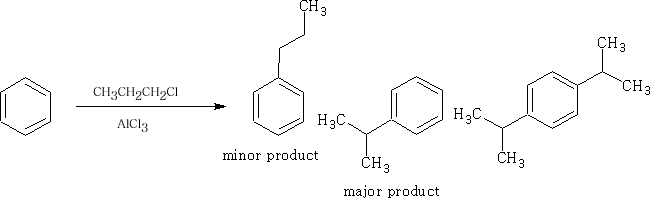
The propyl chloride will rearrange in the presence of the AlCl3.
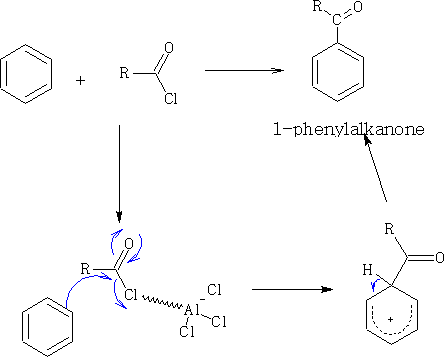
Another reaction with similar details is Friedel-Crafts3 acylation. This is much more reliable and single products are produced. See also Phenols.
3 Price, C.C. Org. React., 1946 , 3 , 1.
It is best to go via the acylation route and then work up the product to get the propyl benzene.
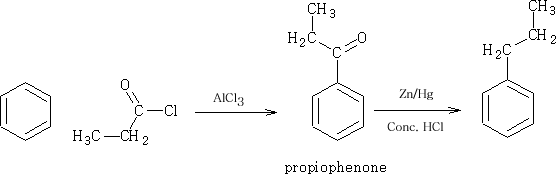
The reduction process using the Zn/Hg and concentrated HCl is known as the Clemmensen Reduction4
4 E. Clemmensen, Ber. 46, 1837 (1913); 47, 51, 681 (1914)
If the reaction is sensitive to acid the best reaction to use in this instance is the Wolff-Kishner reduction5.
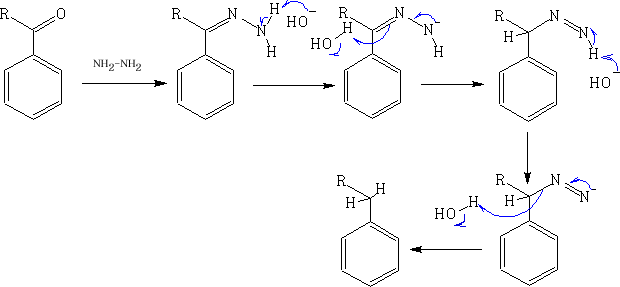
5 N. Kishner, J. Russ. Phys. Chem. Soc. 43, 582 (1911); C.A. 6, 347 (1912); L. Wolff, Ann. 394, 86 (1912)
This reaction is favoured because of the thermodynamic stability of the N2.