Nomenclature:
The ending – al denotes an aldehyde
The ending – one denotes a ketone.
Example:
![]()
The numbering system applies here to ketones: e.g.
![]()
Physical properties:
Aldehydes and ketones are usually colourless, unless an extended system of conjugation is present. The bpt's are higher than those of the corresponding hydrocarbons but lower than those of the corresponding alcohols.
1 The first preparation we have already met: Ozonolysis. (See earlier)
2 Rosenmund Reduction from the acid chloride RCOCl with H2, Pd and Barium Sulphate the following reaction occurs:
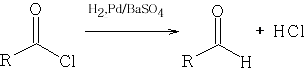
http://www.organic-chemistry.org/namedreactions/rosenmund-reduction.shtm : Organic Reactions Vol IV Chapter 7
The Pd catalyst must be heavily poisoned with the BaSO4 because the reaction will give over-reduction and many by-products will be formed as follows:

3 Jones Oxidation : this reaction uses CrO3 /dil. H2SO4 : this is a mild oxidative method; however, in the case of the aldehyde it will give further oxidation to the carboxylic acid.
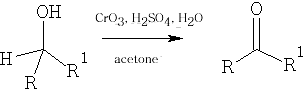
4 Metal Oxidations of Alcohols:
If methanol (CH3OH) is passed over Pt in the presence of Oxygen the following occurs:
![]()
also, ethanol with Nickel or Copper in the presence of oxygen:
![]()
each of these reactions occurs at the surface of the catalyst.
5 Glycol fission: the reagents used in this reaction are Pb(CH3COO)4 and HIO4
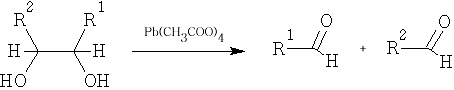
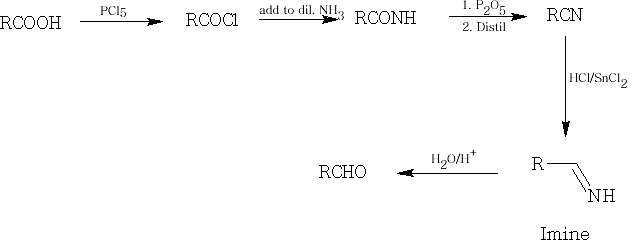
6 Reduction: the reduction of an acid to an aldehyde is quite difficult to accomplish, it is usually done in a roundabout way:
![]()
First of all the acid is converted into an acid chloride with PCl5 and the reduction can than occur with H2 with a Pt catalyst.
Also, we can treat the acid with Lithium Aluminium Hydride (LiAlH4) to form the primary alcohol and then oxidise this into the aldehyde. This is known as the Rosenmund reduction : see earlier.
The last of the reductions is the Stephen Reduction and involves preparing the nitrile from the acid and then using Tin (II) Chloride in HCl to form the imine which is immediately followed by oxidation to the aldehyde using dilute acid.
http://www.organic-chemistry.org/namedreactions/jones-oxidation.shtm : E. R. A H. Jones et al., J. Chem. Soc. 1953, 457, 2548, 3019
H. Stephen, J. Chem, Soc. 127, 1874 (1925): F. D. Bellamy and K. Ou (1984). "Selective reduction of aromatic nitro compounds with stannous chloride in non acidic and non aqueous medium". Tetrahedron Letters 25 (8): 839-842. (1984)
C-C bond formation
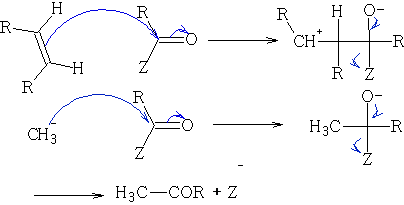
i) Reactions of acid derivatives with organometallic reagents based on the reactions shown in outline above.
![]()
The RMgBr behaves as R - .
![]()
Other organometallic compounds can be used: e.g. RLi
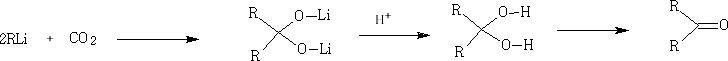
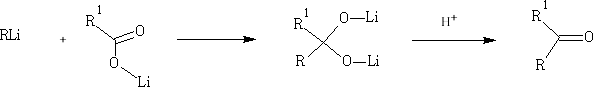
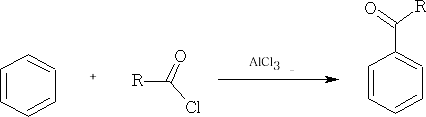
In these reactions the nucleophile is an aromatic substrate or an alkene. The electrophile is an acid chloride or anhydride, usually activated by a Lewis acid
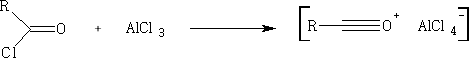
Other common catalysts are SnCl2, ZnCl2 , BF3, HF, H2SO4 , and H3PO4
Aromatic compounds will be dealt in full in a later chapter. G. A. Olah and S. J. Kuhn, J. Org. Chem. 29, 2317 (1964)
With alkenes, we get Markownikov addition:
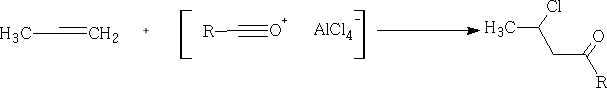
With aromatic compounds, it only works where there are no electron withdrawing substituents. e.g.
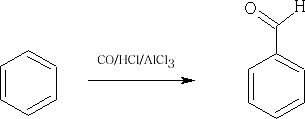
A similar reaction is the Gattermann/Koch reaction:
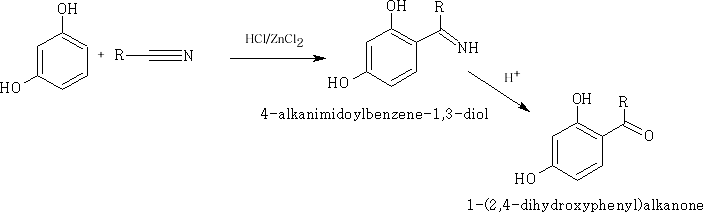
Phenols are highly activated compounds the -OH group pushes electrons into the ring system making certain positions more reactivee.g. the Houben-Hoesch reaction:
The above reaction does not work very well with monohydric alcohols.
The Gattermann reaction : This reaction involves the addition of HCN to the aromatic ring system.
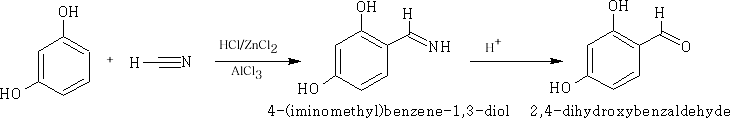
Mechanism: The mechanism below uses the phenolic ether to achieve the addition of the -CHO group, a complex of [AlCl3 ]- [NCH]+ is formed and a SE Reaction ensues at the 4-position on the benzene ring
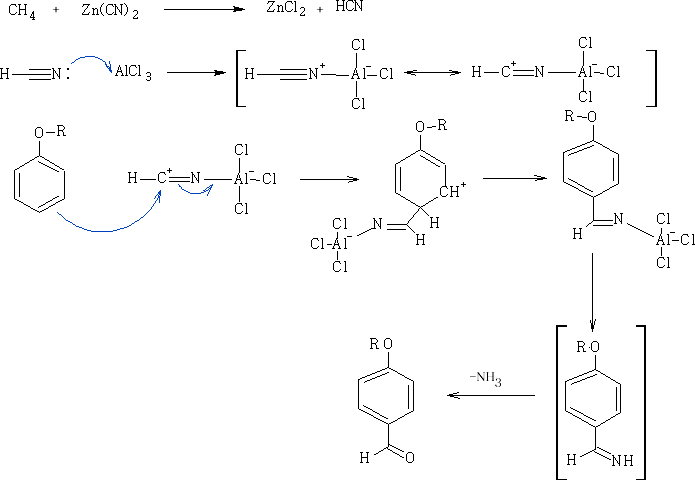
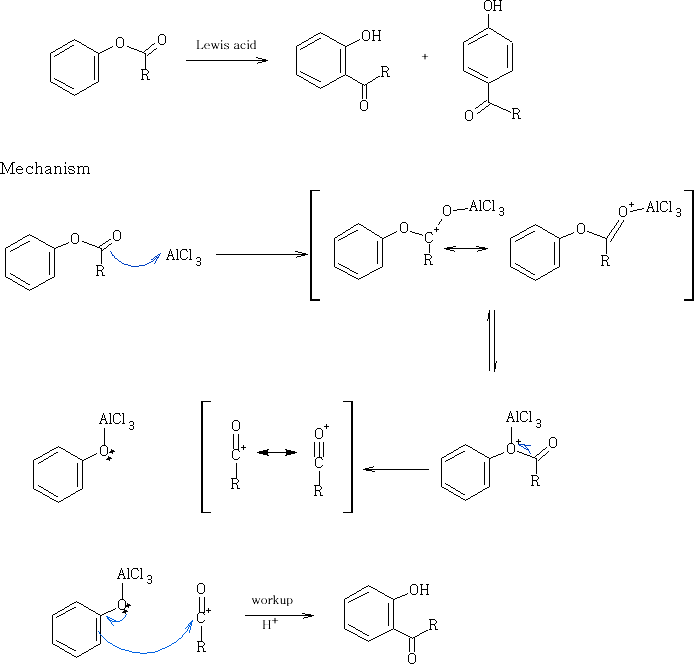
This reaction can be done using a more environmentally friendly acid, methane sulphonic acid CH3SO3H (see footnote 18.)
iii) Esters: Claisen condensation
If ethyl ethanoate is treated with sodium ethoxide the following reaction proceeds:
![]()
Mechanism:
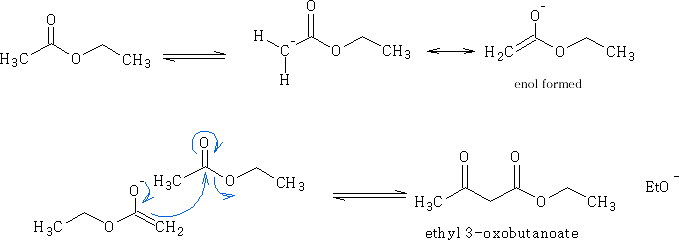
Two molecules of the ethyl ethanoate react together one in the enol form and the other as the ester; the molecules join together as shown above.
a ß-keto ester is formed and is a very important synthetic intermediate.
ß-keto esters with dilute aqueous bases:
![]()
a ketone is formed.
ß-keto esters with a concentrated base:
![]()
a carboxylic acid is formed. This process is “ß-diketone cleavage”.
iv) Rearrangement reactions
The pinacol-pinacolone rearrangement:
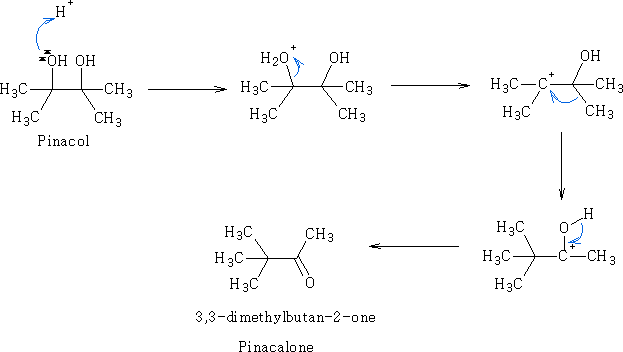
In the course of this reaction, protonation of one of the -OH groups occurs and water leaves to give a carbocation. If both the -OH groups are not alike, then the one which yields a more stable carbocation participates in the reaction. Subsequently, an alkyl group from the adjacent carbon migrates to the carbocation centre. The driving force for this rearrangement step is believed to be the relative stability of the resultant oxonium ion, which has complete octet configuration at all centres (as opposed to the preceding carbocation). The migration of alkyl groups in this reaction occurs in accordance with their usual migratory aptitude, i.e. Ph- > 3-alkyl > 2-alkyl >1-alkyl> H.
This is a general reaction and is usually the procedure for the preparation of ketones of the type:
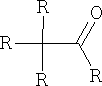
Reactions of aldehydes and ketones:
Consider at this point only those reactions which take place at the carbonyl group.
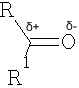
Reactions which involve positions alpha to the carbonyl group will be considered later.
Consider the reaction:
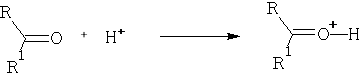
Protonation occurs on the oxygen atom.
The importance of this reaction is that it leads to acceleration of nucleophilic attack on the carbon atom. This is because the +ve charge will be removed on reaction. It is also an important consideration in acid catalysed reactions of aldehydes and ketones which involve the “enol” form of the carbonyl. e.g.
Effect of Nucleophile on carbon:
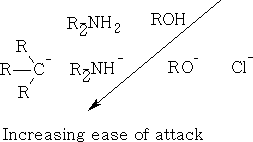
Effect of structure of the C=O group on the nucleophilic attack at this carbon::
The carbon atom goes from trigonal (sp2 ) to tetrahedral (sp3 ) so the sizes of the R groups attached to the carbonyl group will make a difference to the reactivity.
Thus reactions are easier in the order: H2CO > RCHO >R2CO
What the group consists of will also have an effect on the ease with which a reaction will proceed.
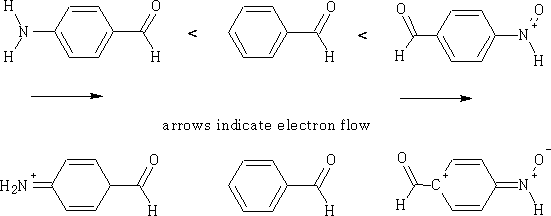
For each of the compounds you can see the effects of the electron flow, the most reactive is going to be the nitro derivative.
Addition of HX :
In a general, three possible reaction situations are going to form:
a) Equilibrium favours R2C=O
b) Equilibrium favours R2CH(OH)
c) R2C(OH)X may undergo further reactions
Formation of C-Hal bonds
Only used for Cl:
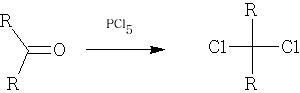
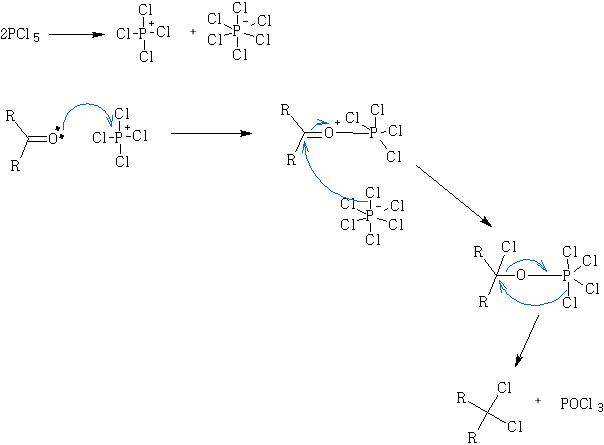
Mechanism:
Formation of C-Oxygen bonds
With water:
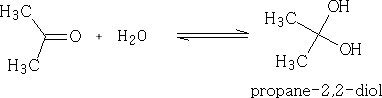
The “hydrates” of aldehydes and ketones are usually very unstable and difficult to isolate. There are some exceptions however;
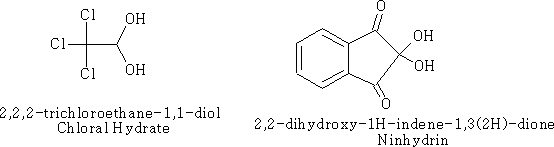
Many aldehydes are largely hydrated or even completely hydrated in aqueous solution.
With alcohols:
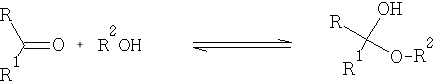
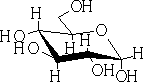
The hemiacetals and –ketals are similar to those of the hydrates i.e. in general very unstable, and the equilibrium lies to the left. One very important exception is where a 5 or 6 member cyclic hemi-acetal or ketal can be formed, as for example in the carbohydrates: e.g. Glucose
Though the hemiketals and hemiacetals are unstable they are easily converted to ketals and acetals in dilute acid solution. This process requires excess of the alcohol, and necessitates continuous removal of the water formed, thus displacing the equilibrium:
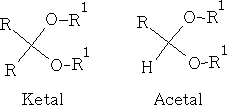
E.g. look at the reaction shown below:
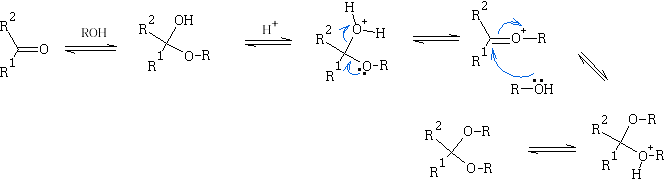
Ketals and acetals are very useful substances, they are stable to base, and to nucleophilic attack in general. Treatment with aqueous acid, however, regenerates the carbonyl group by reversing the above equilibrium. N.b formation of ketals as stated before is favoured when a 5 or 6 membered ring is formed. e.g.
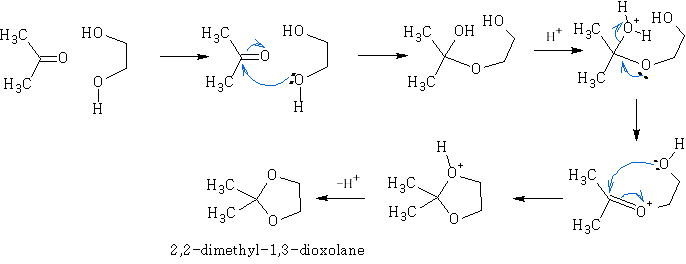
With anhydrides:
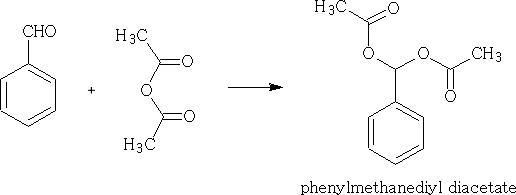
Can you work out the mechanism for this reaction?
Self-condensation:
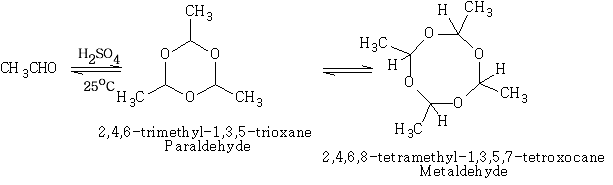
This is an important reaction leading to aldehyde polymers. It is usually acid catalysed.
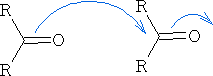
Methanal:
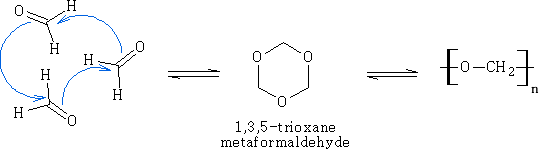
Ethanal:
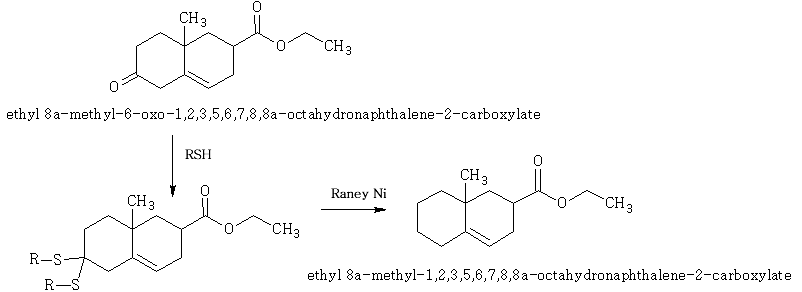
Formation of C – Sulphur Bonds
With mercaptans (thioalcohols):
In these reactions the equilibrium lies to the right. The products are stable to both acid and alkali and are very useful as a means of protecting a carbonyl group during a reaction. Regeneration of the carbonyl group is easily accomplished by treatment with salts of Hg2+ or Cd2+ .
These compounds have some interesting uses:
e.g.
This is an important method by which carbonyls are reduced.
E.g.
With bisulphites:
The addition are formed from aldehydes, methyl ketones and some 5 and 6 cyclic ketones. Alpha substituted ketones don't give adducts because of steric interactions.
Dilke, Eley, J. Chem. Soc. 1949, 2601, 2613
K. Hoesch, Ber. 48, 1122 (1915); J. Houben, ibid. 59, 2878 (1926).
Y. Sato, M. Yato, T. Ohwada, S. Saito and K. Shudo* Heterocycles , 51 , 2163. (1999) , J. Am. Chem. Soc. 1995 , 117 , 3037. , J. Heterocyclic Chem. 1971 , 8 , 129.
23 Fries rearrangement in methane sulphonic acid, an environmental friendly acid. A. Commarieu, W. Hoelderich , J. A. Laffitte, M.-P. Dupont, J. Mol. Cat. A.: Chemical, 2002 , 182-183 , 137-141.:
Fries, K.; Finck, G. Ber., 1908 , 41 , 2447. :Fries, K.; Pfaffendorf, W. Ber. , 1910 , 43 , 212.
http://www.organic-chemistry.org/namedreactions/claisen-condensation.shtm