Once, during Prohibition, I was forced to live for days on nothing but food and water.
W.C. Fields
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in beer.
Dave Barry
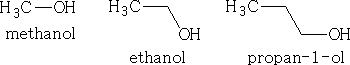
The functional group is ľOH and in a compound is known as the hydroxy group. If the main group in the molecule is the OH then the compound is an alcohol. The name is given as for the alkane but with the ending ľol,
e.g.

or, if the OH is not on the 1 position it will -2-ol, or -3-ol depending on the carbon atom it is attached to.
e.g.
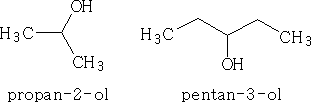
If the OH group is not the main group in the molecule then the OH may be called hydroxy .
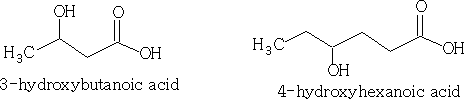
Alcohols like the halogenalkanes are defined as primary (1o), secondary (2o) and tertiary (3o) depending on the position of the ľOH group.
Primary: RCH2OH
Secondary: R2CHOH
Tertiary: R3COH
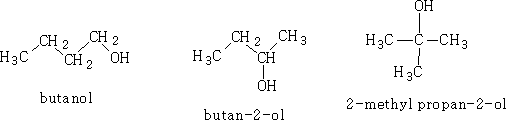
Butanol can exist in all three forms, 1o,2o and 3o ;please note the naming!
The polarisation of the Cδ+ -Oδ- bond is qualitatively similar to the C-Hal bond. There is the possibility of nucleophilic attack at the Carbon, and electrophilic attack at the Oxygen. Alcohols also show the reactions of the ľOH group.
Physical properties
Hydrogen bonding raises the mpt and bpt's of alcohols far above those of hydrocarbons of similar molecular weight. The hydroxyl group renders low molecular weight alcohols water soluble because the -OH group forms hydrogen bonds with the water, the higher alcohols become insoluble as the hydrophobic properties of the alkane end of the molecule kicks in.
Chemical properties
With the ľOH group we might expect the group to ionise as follows ľO- + H+ , as it does in HCl, but it does not do this, so we need a way to quantify the ľOH group and any other acid. e.g.
![]()
a strong acid will have its equilibrium over to the RHS of the equation.
Acidity of OH:
![]()
Alcohols are very much weaker acids than water, e.g. ethanol pKa about 18
(N.b. the smaller the pKa value the stronger the acid). Water has a pKa of about 16
Salts (alkoxides) are formed by reaction of alcohol with alkali metal or alkaline earth metals:
1. With sodium: the alcohols give up a proton, and in this capacity they behave as an acid, forming a salt-sodium alkoxide.
![]()
2. Alcohols will react with even weaker acids: e.g. NH2-, H-
![]()
![]()
3. Oxidation to form the C=O group:
Primary alcohols form first the aldehyde then on further oxidation the carboxylic acid. The oxidant is acidified potassium dichromate (VI).![]()
With refluxing, further oxidation gives:
![]()
Secondary Alcohols are oxidised differently with acidified K2Cr2O7, this time ketones are formed:
![]()
4. Dehydration: This is the elimination of water from the molecule. It is achieved by passing the alcohol vapour over aluminium oxide at 400oC.
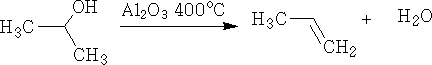
Dehydration can also be done using concentrated H2SO4, e.g.
At 180oC ethene is formed.
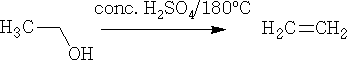
At 145oC diethyl ether is formed.
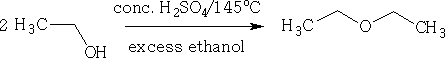
5. Formation of Esters
a)
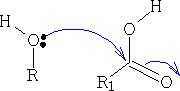
This reaction is very slow, to help the process a catalyst is used, this enables the alcohol to become a better nucleophile and for the acid to be a better electrophile. The catalyst is usually an acid, this protonates the carbonyl oxygen and thus increases the electrophilicity of the acid.
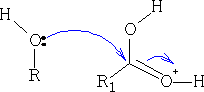
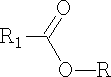
The ester that is formed is as shown below, you can see that the original C=O has reformed into the carbonyl group and water was lost; try to work out the mechanism for this resultant.
If too much acid is used in this esterification, the reaction will go much slower as the acid will protonate most of the ROH. Concentrated H2SO4 or dry HCl ( Fischer Speier1) are the most common used catalysts.
b) Basic catalysts, to give RO- , can be used; i.e. they increase the nucleophilicity of the alcohol. These cannot be used with free carboxylic acids, as they would react to give the salts, but are used with acid chlorides. This is the Schotten Baumann2 reaction.
1 E. Fischer and A. Speier, Ber. 28, 3252 (1895).
2 Bull. Chem. Soc. Japan 42, 1756 (1969)
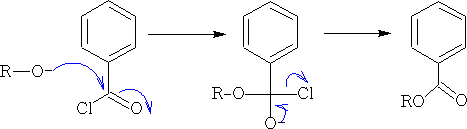
1. Alcohols with a strongly electrophilic carbon:
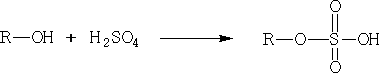
The alcohol can now attack the R group:
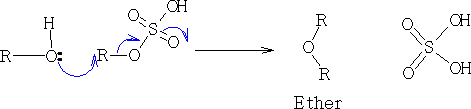
2. RO- as a strong nucleophile: Williamson synthesis3
3 A. W. Williamson, J. Chem. Soc. 4, 229 (1852): also, L. F. Fieser and M. Fieser, Advanced Organic Chemistry, pp 113, 306 ( New York 1961).
![]()
Preparation of Alcohols
1. A Sn reaction by HO- on R-Hal or CH3COO-
![]()
2. Grignard Reagents: RMgX
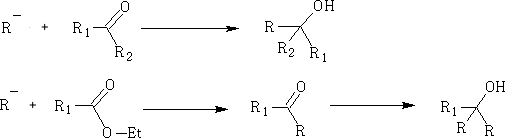
3. Reduction of aldehydes and ketones: this is done using H2/Pt, LiAlH4 and NaBH4 (these being a source of H-)
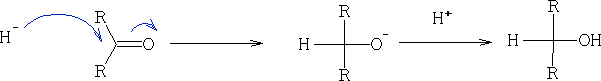
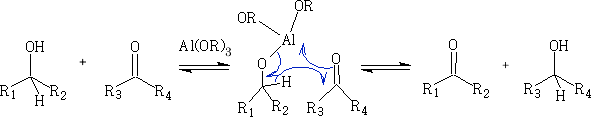
This reaction uses an aluminium catalysed H - shift from the CH3CH(OH)CH3 (propan-2-ol) via a 6 membered transition state. The catalyst is usually Al(OiPr)3 (OiPr is propan-2-ol) and is used because one of the products is propanone and this can be removed from the mixture by continuous distillation.
4 Reduction of acids and esters with LiAlH4: